Preventing Corrosion Damage in Metals
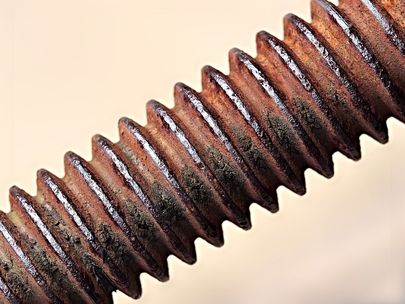
Corrosion has a number of negative effects on your business and its operations. When metal structures corrode, they can endanger the safety of your employees. Other corrosion instances can lead to structure collapses and increased risks of injuries onsite. Damage from corrosion can also be expensive to fix – the smallest signs of corrosion may require costly repairs and maintenance.
There are a number of ways you can prevent corrosion damage in metals. Here are some of them:
Metal Selection
One of the quickest ways to prevent corrosion is to switch your existing metal components to corrosion-resistant types such as stainless steel or aluminium. These kinds of metals reduce the need for additional corrosion protection.
Environmental Modification
Many environmental factors impact the likelihood of corrosion: the rise and fall of temperature, air quality, the presence of electrolytes in the water and more. You can try simple solutions such as reducing the metals to rain or seawater or implement more complex measures like decreasing the amount of oxygen, sulphur and chlorine in the surrounding area. By controlling the conditions of the environment, you minimise the effects of corrosion.
Protective Coating
Barrier coatings are heated or sprayed onto the metal surface to create a protective thin film. This layer acts as a shield that prevents corrosive compounds from creating electrochemical charges.
Common organic coatings for metal include:
- Powder coatings
- Water-soluble coatings
- High-solid coatings
- Acrylic, styrene or vinyl polymer latex coatings
- Alkyd and epoxy ester coatings
- Two-part urethane coatings
Cathodic Protection
Another way you can prevent metal corrosion from occurring is to apply an opposing electrical current to the metal’s surface, the process of which is called cathodic protection. By using an outside course of electrical current, you can overpower a corrosive current from damaging metal.
One cathodic protection procedure that Tim Keane from Plastico Industries suggests is to utilise a sacrificial anode. This involves fastening a tiny, reactive metal onto the part of the metal you wish to protect from corrosion. Metal ions will flow from the reactive metal to the less active area, reducing corrosion in the process.
Your Partner in Construction
At Timberfix, we have a wide range of stainless steel and aluminium that provide excellent resistance to corrosion. We are a dependable construction material supplier that offers top-quality products at competitive prices.
Since we are a family business of 3rd generation builders, we have developed an extensive knowledge of the construction sector and have studied the industry’s demands, making sure our products target your requirements, however complex.
Our company understands the importance of Service. We employ hard-working and helpful staff who are more than willing to help you with all your construction needs. Our team specialises in quick deliveries through Sydney, Blue Mountains and New South Wales.
Let us know what you need. Get in touch by emailing us at sales@timberfix.com.au or calling us on 1300 888 729.

